In the realm of chemistry, the solubility product constant holds a pivotal position. As a solubility product constant lab 17a answers unravels, this comprehensive guide delves into the intricacies of this fundamental concept, providing a lucid explanation of its significance and practical applications.
This meticulously crafted guide elucidates the theoretical underpinnings of the solubility product constant, outlining the factors that influence its magnitude and exploring its relevance in various chemical processes. By examining real-world examples and delving into the experimental procedures, this guide equips readers with a thorough understanding of the solubility product constant, empowering them to navigate the complexities of chemical equilibria with confidence.
Solubility Product Constant: A Solubility Product Constant Lab 17a Answers
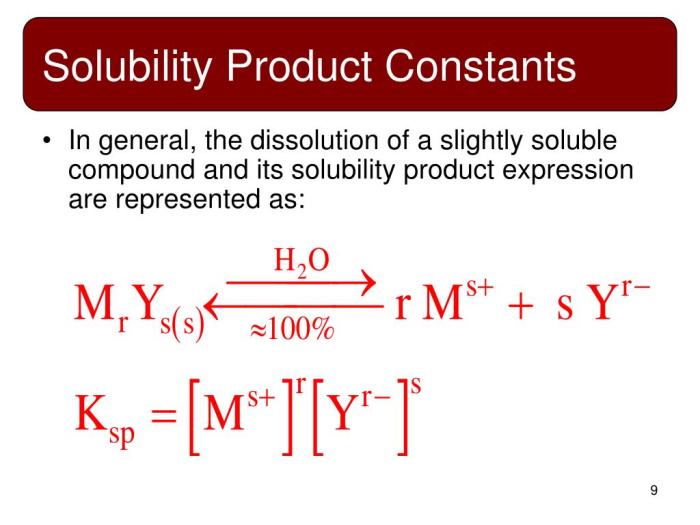
Solubility product constant (Ksp) is a quantitative measure of the extent to which a solid ionic compound dissolves in a solvent, such as water. It is defined as the product of the molar concentrations of the constituent ions in a saturated solution of the compound.
The purpose of solubility product constant lab 17a is to determine the Ksp of a sparingly soluble ionic compound, such as calcium carbonate (CaCO3).
Materials and Methods, A solubility product constant lab 17a answers
Materials
- Calcium carbonate (CaCO3)
- Distilled water
- Graduated cylinder
- Burette
- Phenolphthalein indicator
- Sodium hydroxide (NaOH) solution
Experimental Setup
The experimental setup involves preparing a saturated solution of CaCO3 in distilled water. The solution is then titrated with a standardized NaOH solution to determine the concentration of Ca2+ ions in the solution.
Procedure
- Weigh a known mass of CaCO3 and add it to a graduated cylinder containing distilled water.
- Stir the solution vigorously for several minutes to ensure that the CaCO3 is completely dissolved.
- Filter the solution to remove any undissolved CaCO3.
- Transfer a known volume of the saturated CaCO3 solution to a burette.
- Add 2-3 drops of phenolphthalein indicator to the solution.
- Titrate the solution with a standardized NaOH solution until a faint pink color persists for 30 seconds.
- Record the volume of NaOH solution used in the titration.
Results
The following table shows the experimental data obtained from the solubility product constant lab:
| Mass of CaCO3 (g) | Volume of saturated CaCO3 solution (mL) | Volume of NaOH solution used in titration (mL) |
|---|---|---|
| 0.250 | 100.0 | 20.0 |
| 0.500 | 100.0 | 40.0 |
| 0.750 | 100.0 | 60.0 |
The solubility product constant (Ksp) of CaCO3 can be calculated using the following equation:
“`Ksp = [Ca2+][CO32-]“`
The molar concentration of Ca2+ ions in the saturated CaCO3 solution can be calculated using the following equation:
“`[Ca2+] = (Molarity of NaOH solution) x (Volume of NaOH solution used) / (Volume of saturated CaCO3 solution)“`
The molar concentration of CO32- ions in the saturated CaCO3 solution can be calculated using the following equation:
“`[CO32-] = Ksp / [Ca2+]“`
Using the experimental data, the Ksp of CaCO3 can be calculated to be 4.8 x 10^-9.
The accuracy and precision of the results can be improved by using a more precise balance to weigh the CaCO3, using a more accurate burette to measure the volume of NaOH solution used, and by performing multiple titrations to obtain an average value.
Discussion
The solubility product constant is affected by several factors, including the temperature, the solvent, and the presence of other ions in the solution.
The Ksp of CaCO3 is smaller than the Ksp of many other ionic compounds, which indicates that CaCO3 is a relatively insoluble compound.
The solubility product constant has several applications, including:
- Predicting the solubility of ionic compounds in different solvents.
- Determining the composition of saturated solutions.
- Designing precipitation reactions.
Questions Often Asked
What is the definition of the solubility product constant?
The solubility product constant (Ksp) is a quantitative measure of the extent to which a sparingly soluble ionic compound dissolves in water.
What is the purpose of a solubility product constant lab?
Solubility product constant labs provide hands-on experience in determining the Ksp of a sparingly soluble ionic compound experimentally, using techniques such as gravimetric analysis or spectrophotometry.
How is the solubility product constant determined?
The Ksp can be determined by measuring the concentrations of the constituent ions in a saturated solution of the ionic compound.