Section 2.1 classifying matter answer key – Embark on a journey through Section 2.1: Classifying Matter, where we unravel the fundamental principles that govern the nature and composition of matter. Delving into the criteria for classifying matter, we will explore the distinct characteristics of elements, compounds, and mixtures, providing a comprehensive understanding of the building blocks of our universe.
As we delve deeper into this section, we will uncover the intricate relationships between the properties and behavior of matter. From physical attributes like density and melting point to chemical properties such as reactivity and flammability, we will gain insights into how these properties shape the identity and applications of different substances.
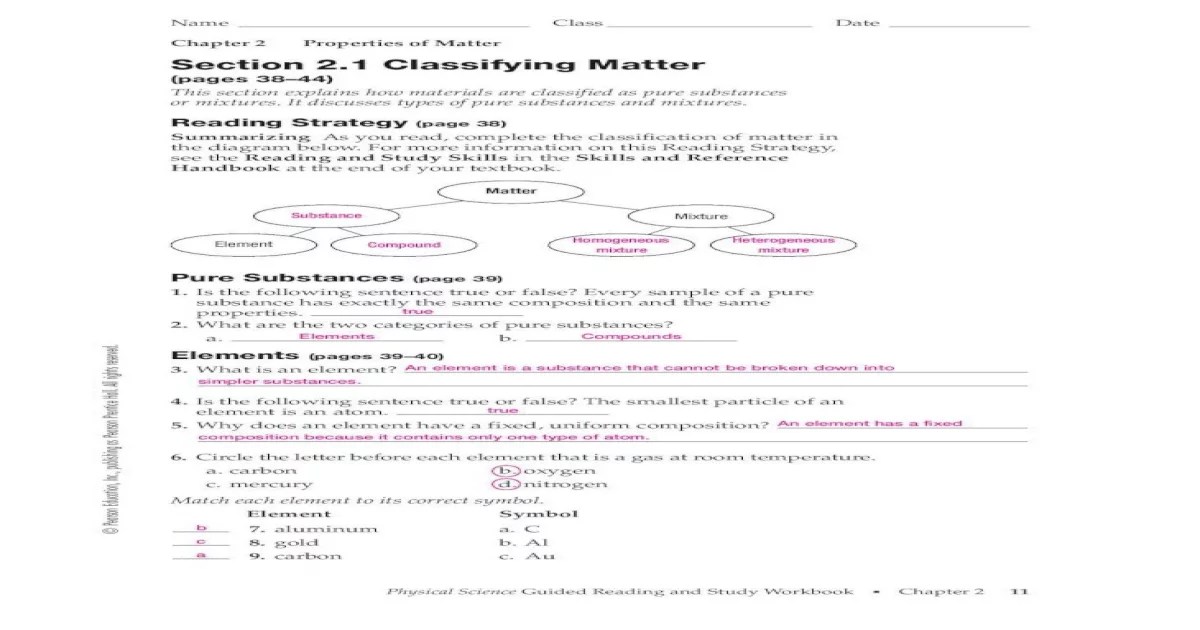
2. Classifying Matter: Section 2.1 Classifying Matter Answer Key
Matter is anything that has mass and takes up space. It can exist in different states, such as solid, liquid, or gas. The basic properties of matter include mass, volume, density, and temperature.
Criteria for Classifying Matter, Section 2.1 classifying matter answer key
Matter can be classified based on its composition and chemical properties. The main criteria for classifying matter are:
- Composition:Matter can be classified as an element, compound, or mixture.
- Chemical properties:Matter can be classified based on its reactivity, flammability, and other chemical properties.
Detailed FAQs
What is the significance of classifying matter?
Classifying matter allows us to organize and understand the vast array of substances in the world, enabling us to predict their properties and behavior based on their composition and structure.
How do we differentiate between elements, compounds, and mixtures?
Elements are pure substances that cannot be broken down into simpler substances by chemical means, while compounds are pure substances composed of two or more elements chemically combined in fixed proportions. Mixtures, on the other hand, are combinations of two or more substances that retain their individual identities and can be separated by physical means.
What are some examples of physical and chemical properties of matter?
Physical properties include density, melting point, boiling point, and color, while chemical properties include reactivity, flammability, and acidity.