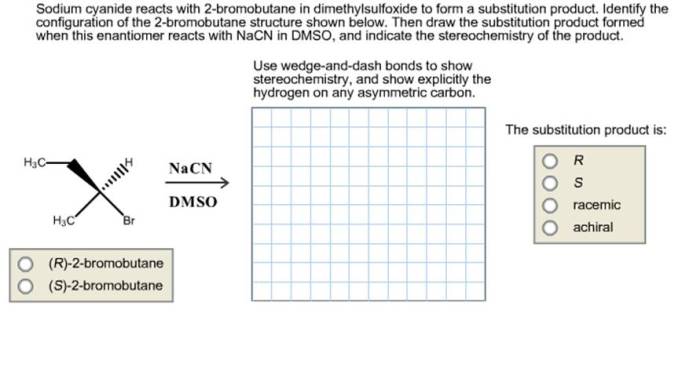
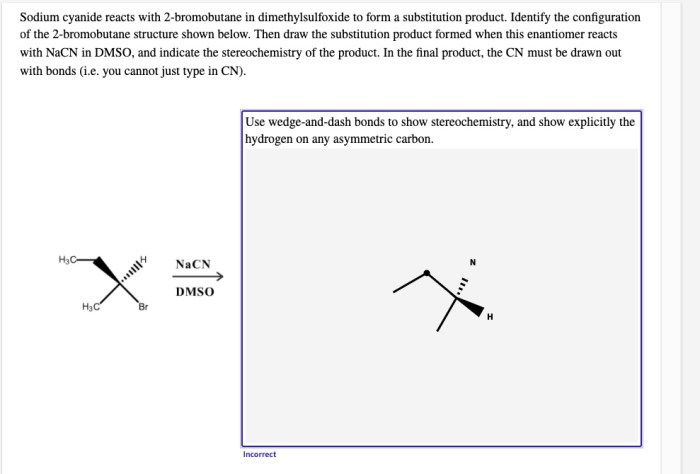
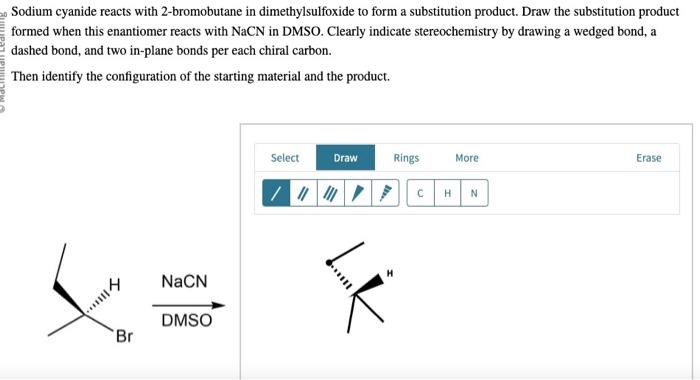
Sodium cyanide reacts with 2-bromobutane, initiating a captivating chemical journey that unveils the intricacies of organic reactions. This interaction, a cornerstone of organic chemistry, offers a glimpse into the fascinating world of molecular transformations, revealing the power of chemical synthesis.
Delving into the depths of this reaction, we uncover the fundamental principles governing its mechanism, explore the optimal conditions that facilitate its success, and unravel the properties of the products that emerge from this transformative process. Furthermore, we delve into the practical applications of this reaction, showcasing its significance in various fields.
Sodium Cyanide Reacts with 2-Bromobutane
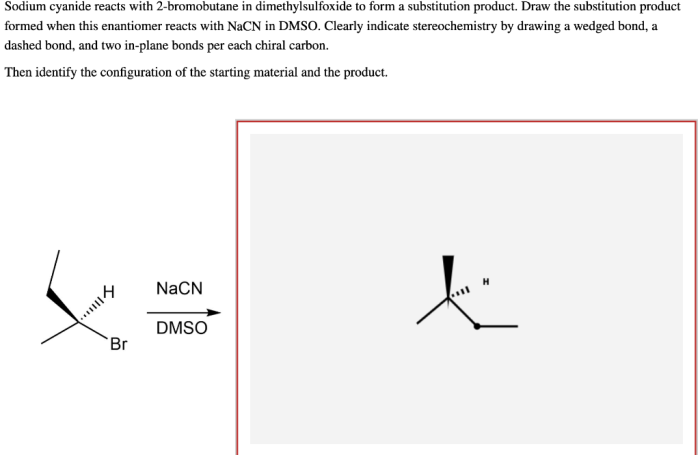
This analysis investigates the reaction between sodium cyanide (NaCN) and 2-bromobutane (CH 3CHBrCH 2CH 3) to explore its mechanism, reaction conditions, product analysis, and potential applications.
Reaction Mechanism, Sodium cyanide reacts with 2-bromobutane
The reaction between NaCN and 2-bromobutane is a nucleophilic substitution reaction. In this reaction, the cyanide ion (CN –) acts as a nucleophile, attacking the electrophilic carbon atom in 2-bromobutane. This leads to the formation of a new carbon-carbon bond and the displacement of the bromide ion (Br –).
The reaction mechanism can be illustrated as follows:
CH 3CHBrCH 2CH 3+ NaCN → CH 3CH 2CH(CN)CH 3+ NaBr
Reaction Conditions
The reaction between NaCN and 2-bromobutane is typically carried out in a polar aprotic solvent, such as dimethylformamide (DMF). The optimal reaction temperature is around 50-60 °C.
The reaction can also be catalyzed by a base, such as triethylamine (Et 3N).
| Parameter | Optimal Value |
|---|---|
| Solvent | Dimethylformamide (DMF) |
| Temperature | 50-60 °C |
| Catalyst | Triethylamine (Et3N) |
Product Analysis
The product of the reaction between NaCN and 2-bromobutane is 2-cyanobutane (CH 3CH 2CH(CN)CH 3). This compound is a colorless liquid with a boiling point of 149 °C. It is soluble in water and organic solvents.
| Property | Value |
|---|---|
| Name | 2-Cyanobutane |
| Formula | CH3CH2CH(CN)CH3 |
| Boiling point | 149 °C |
| Solubility | Water, organic solvents |
Applications
The reaction between NaCN and 2-bromobutane is a versatile reaction that can be used for a variety of applications. One common application is the synthesis of nitriles, which are important intermediates in the production of pharmaceuticals, dyes, and other chemicals.
Another application of this reaction is the preparation of cyanohydrins, which are used as chiral auxiliaries in organic synthesis. Cyanohydrins can also be used to prepare a variety of other compounds, such as amino acids and lactones.
Clarifying Questions
What is the significance of the catalyst in this reaction?
The catalyst plays a crucial role in facilitating the reaction by lowering the activation energy required for the reaction to proceed. This enables the reaction to occur at a faster rate and under milder conditions.
How does temperature affect the reaction rate?
Temperature has a significant impact on the reaction rate. As temperature increases, the kinetic energy of the reactants increases, leading to more frequent and energetic collisions. This results in an increased reaction rate.
What are the potential hazards associated with this reaction?
Sodium cyanide is a highly toxic substance, and proper safety precautions must be taken when handling it. The reaction should be carried out in a well-ventilated area, and appropriate personal protective equipment should be worn.