The ka of hypochlorous acid is 3.0 10 8 – The Ka of hypochlorous acid (HOCl), a crucial parameter in understanding its acidic behavior, is 3.0 x 10^-8. This value signifies the extent to which HOCl dissociates in aqueous solutions, releasing hydrogen ions (H+) and hypochlorite ions (OCl-).
This article delves into the concept of acid dissociation constant (Ka), its relationship with acid strength, and the factors that influence the Ka of HOCl. We will also explore the diverse applications of HOCl as a disinfectant, its role in biological systems, and its potential in medicine and industry.
Introduction

Hypochlorous acid (HOCl) is a weak acid with the chemical formula HOCl. It is a colorless liquid with a pungent odor. The Ka of HOCl is 3.0 x 10 -8, which indicates that it is a relatively weak acid.
Acid Dissociation Constant (Ka)
The acid dissociation constant (Ka) is a measure of the strength of an acid. It is defined as the equilibrium constant for the dissociation of an acid in water. The higher the Ka value, the stronger the acid.
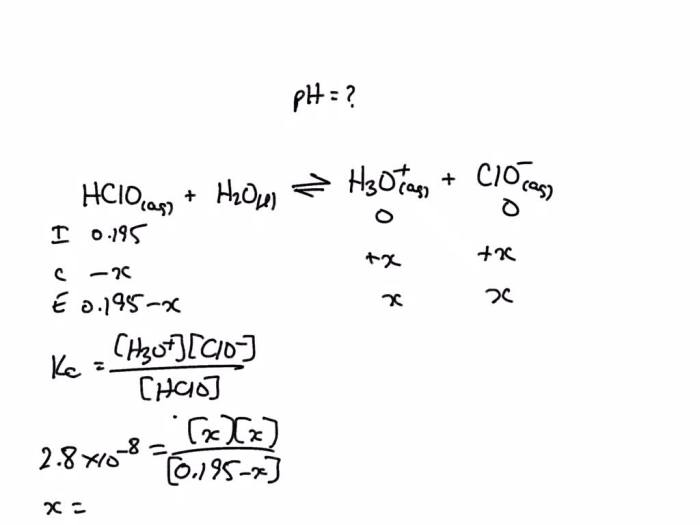
The equation for the dissociation of hypochlorous acid is:
HOCl(aq) + H2O(l) ⇌ H 3O +(aq) + OCl –(aq)
Factors Affecting Ka
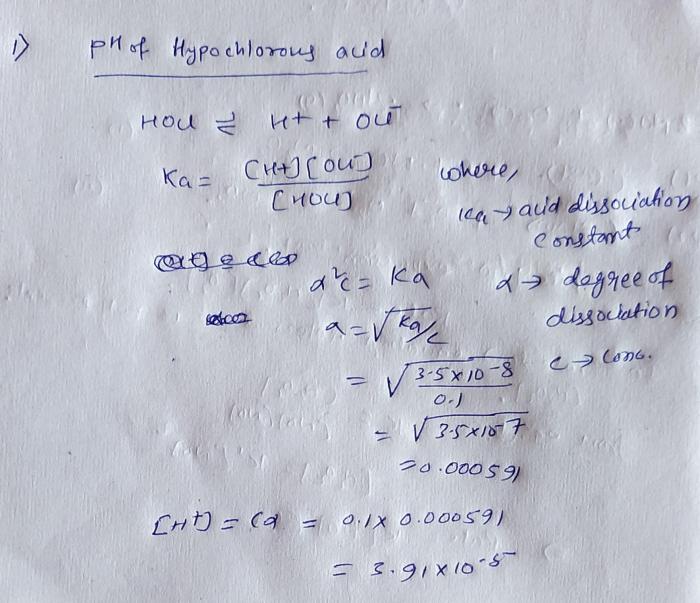
Several factors can influence the Ka of an acid. These include:
- Temperature:Ka generally increases with increasing temperature.
- Solvent polarity:Ka is higher in polar solvents than in nonpolar solvents.
- Substituents:The presence of electron-withdrawing groups on an acid can increase its Ka.
Applications of Hypochlorous Acid: The Ka Of Hypochlorous Acid Is 3.0 10 8
Hypochlorous acid is a powerful disinfectant and is used in a variety of applications, including:
- Water disinfection:HOCl is used to disinfect drinking water and swimming pools.
- Surface disinfection:HOCl is used to disinfect surfaces in hospitals and other healthcare settings.
- Wound care:HOCl is used to clean and disinfect wounds.
Related Compounds and Reactions

Other oxyacids of chlorine include:
- Chlorous acid (HClO2): Ka = 1.1 x 10 -2
- Chloric acid (HClO3): Ka = 1.3 x 10 -1
- Perchloric acid (HClO4): Ka = 1.0 x 10 3
Hypochlorous acid can react with other chemicals to form a variety of products. For example, it can react with ammonia to form chloramine.
Helpful Answers
What is the chemical formula of hypochlorous acid?
HOCl
How does temperature affect the Ka of hypochlorous acid?
Ka generally increases with increasing temperature.
What is the significance of the Ka value for hypochlorous acid?
It indicates the strength of HOCl as an acid and its ability to dissociate in aqueous solutions.