Embarking on an exploration of the Bohr Model of Hydrogen Gizmo Answers, this comprehensive guide delves into the intricacies of atomic structure, energy level transitions, and the limitations and applications of this groundbreaking model.
Providing a clear understanding of the Bohr model’s key features, energy level concepts, and the significance of the Rydberg formula, this guide unravels the complexities of atomic physics in an accessible manner.
Bohr Model of Hydrogen Gizmo Overview
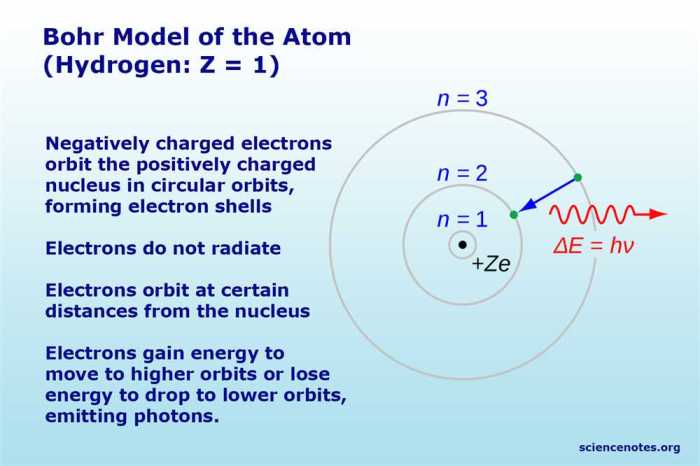
The Bohr Model of Hydrogen Gizmo is an interactive simulation that allows students to explore the structure of the hydrogen atom and its energy levels. It provides a visual representation of the key concepts of the Bohr model, including energy levels, electron transitions, and the emission of light.
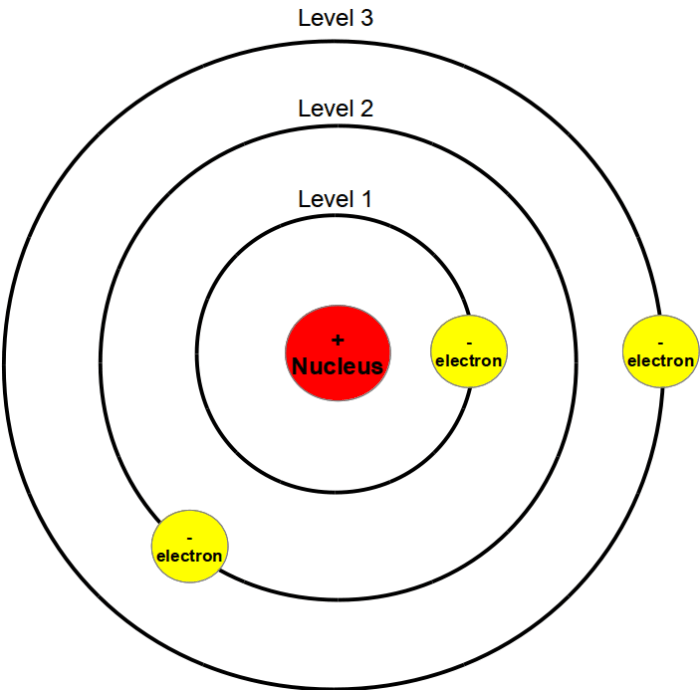
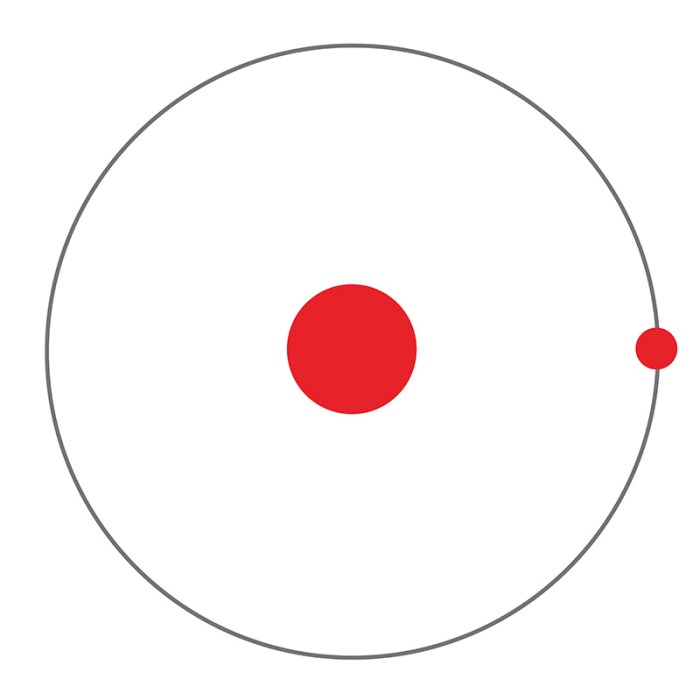
The Gizmo features a hydrogen atom with a nucleus and an electron. The nucleus is fixed at the center, and the electron can move around the nucleus in circular orbits. The Gizmo allows users to change the energy level of the electron and observe the corresponding changes in its orbit and the emission of light.
Energy Levels and Transitions, Bohr model of hydrogen gizmo answers
In the Bohr model, electrons occupy specific energy levels around the nucleus. The energy of each level is quantized, meaning that it can only take on certain discrete values. The lowest energy level is the ground state, and higher energy levels are excited states.
Electrons can transition between energy levels by absorbing or emitting photons of light. When an electron absorbs a photon, it moves to a higher energy level. When an electron emits a photon, it moves to a lower energy level.
Rydberg Formula and Hydrogen Spectrum
The Rydberg formula is an equation that predicts the wavelengths of light emitted by hydrogen atoms when electrons transition between energy levels.
The Rydberg formula is given by: $$ \frac1\lambda = R_H \left(\frac1n_f^2 – \frac1n_i^2\right) $$ where:
- λ is the wavelength of the emitted light
- R_H is the Rydberg constant (1.0973731 x 10^7 m^-1)
- n_f is the final energy level of the electron
- n_i is the initial energy level of the electron
The Rydberg formula can be used to explain the different spectral lines observed in the hydrogen spectrum. The hydrogen spectrum consists of a series of discrete lines at specific wavelengths. Each line corresponds to a transition between two specific energy levels.
Limitations of the Bohr Model
The Bohr model is a simplified model of the hydrogen atom. It does not take into account many of the complexities of the atom, such as the wave-particle duality of electrons and the interactions between electrons.
Despite its limitations, the Bohr model is still a useful tool for understanding the basic structure of the hydrogen atom and the emission of light.
Applications and Extensions
The Bohr model has been applied to a wide variety of problems in physics and chemistry. It has been used to explain the structure of atoms, the emission of light, and the chemical bonding between atoms.
The Bohr model has also been extended to other elements and more complex atomic systems. The extended Bohr model is known as the quantum mechanical model of the atom.
FAQ Explained: Bohr Model Of Hydrogen Gizmo Answers
What is the purpose of the Bohr Model of Hydrogen Gizmo?
The Bohr Model of Hydrogen Gizmo is an interactive simulation designed to help students visualize and understand the energy levels, transitions, and spectrum of the hydrogen atom.
How does the Bohr model explain the hydrogen spectrum?
The Bohr model explains the hydrogen spectrum by proposing that electrons occupy specific energy levels within the atom and that transitions between these levels result in the emission or absorption of photons with specific wavelengths.
What are the limitations of the Bohr model?
The Bohr model has limitations, including its inability to explain the fine structure of spectral lines, the behavior of electrons in atoms with more than one electron, and the wave-particle duality of electrons.