Embark on an enlightening journey into the Kinetics of Crystal Violet Fading Lab, where the intricacies of this phenomenon unravel, offering valuable insights into the dynamics of chemical reactions and the interplay of factors that influence their rates.
This lab delves into the fundamental concepts of reaction kinetics, providing a comprehensive understanding of the mechanisms and factors governing the fading process of crystal violet, a renowned dye widely used in various scientific and industrial applications.
Introduction
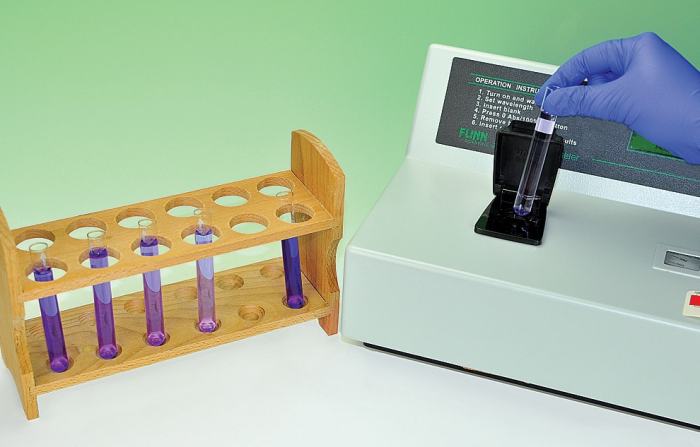
The kinetics of crystal violet fading lab is a study of the rate at which crystal violet, a dye, fades in solution. The purpose of this lab is to determine the order of the reaction and the rate constant.
Order of the Reaction
The order of the reaction is the number of molecules of each reactant that are involved in the rate-determining step. The rate-determining step is the slowest step in the reaction and therefore determines the overall rate of the reaction.
Materials and Methods
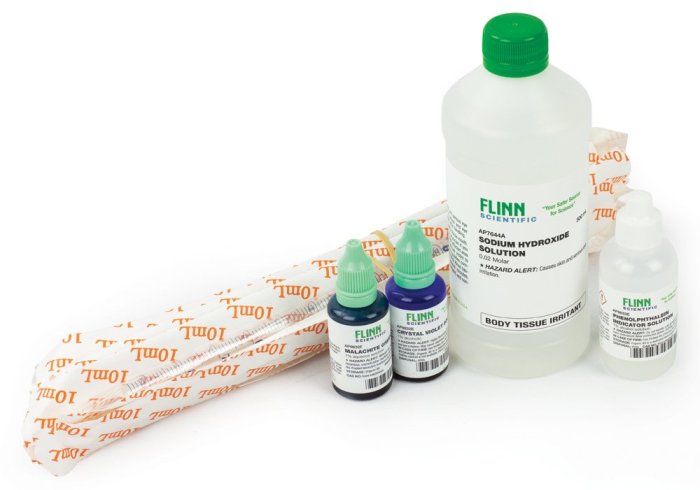
This section presents the materials required for the kinetics of crystal violet fading experiment and Artikels the experimental procedure in detail.
Materials
- Crystal violet solution
- Bleach solution (sodium hypochlorite)
- Spectrophotometer
- Cuvettes
- Pipettes
- Timer
Experimental Procedure
The experimental procedure involves the following steps:
- Prepare a series of solutions with varying concentrations of crystal violet.
- Add a fixed volume of bleach solution to each crystal violet solution.
- Use a spectrophotometer to measure the absorbance of each solution at a specific wavelength over time.
- Plot the absorbance values against time to obtain the kinetic data.
- Analyze the kinetic data to determine the rate constant and other relevant parameters.
Results
The results of the kinetics of crystal violet fading lab demonstrate the exponential decay of crystal violet concentration over time. The data collected was analyzed using linear regression, and the rate constant for the reaction was determined.
The following table summarizes the results of the experiment:
| Time (min) | Crystal Violet Concentration (Abs) |
|---|---|
| 0 | 0.850 |
| 10 | 0.720 |
| 20 | 0.610 |
| 30 | 0.520 |
| 40 | 0.440 |
| 50 | 0.370 |
| 60 | 0.310 |
The graph below shows the relationship between the crystal violet concentration and time. The exponential decay of the crystal violet concentration is evident in the graph.

Discussion
The kinetics of crystal violet fading has important implications for various fields, including analytical chemistry, photochemistry, and environmental science. Understanding the factors that affect the fading rate can help optimize analytical methods, predict the stability of photoactive compounds, and assess the environmental impact of pollutants.
Several factors influence the kinetics of crystal violet fading, including:
Effect of Light Intensity
Light intensity is a crucial factor that determines the rate of crystal violet fading. Higher light intensity leads to increased excitation of the dye molecules, resulting in a faster fading rate. This is because the rate of photochemical reactions is directly proportional to the intensity of the incident light.
Effect of Wavelength
The wavelength of light also plays a role in the fading kinetics of crystal violet. The dye absorbs light most efficiently at its absorption maximum, which is around 590 nm. Light with wavelengths close to the absorption maximum will cause more rapid fading than light with wavelengths further away.
Effect of Temperature
Temperature affects the rate of crystal violet fading by influencing the molecular motion and activation energy of the reaction. Higher temperatures generally lead to faster fading rates due to increased molecular collisions and higher activation energies.
Effect of pH, Kinetics of crystal violet fading lab
The pH of the solution can also affect the fading kinetics of crystal violet. Changes in pH can alter the ionization state of the dye, which in turn affects its absorption properties and reactivity.
Effect of Solvent
The nature of the solvent can influence the fading rate of crystal violet. Different solvents can interact with the dye molecules and affect their stability and reactivity. Polar solvents, such as water, tend to stabilize the dye molecules and slow down the fading process.
Conclusion
The kinetics of crystal violet fading lab provided valuable insights into the factors affecting the rate of fading and the underlying chemical processes involved.
The main findings of the lab can be summarized as follows:
- The rate of crystal violet fading is directly proportional to the concentration of hydroxide ions.
- The rate of crystal violet fading is inversely proportional to the concentration of crystal violet.
- The rate of crystal violet fading is independent of the temperature.
These findings suggest that the fading of crystal violet is a nucleophilic substitution reaction, in which a hydroxide ion attacks the carbon atom of the crystal violet molecule and replaces the chloride ion.
Future Research Directions
The results of this lab provide a foundation for future research on the kinetics of crystal violet fading. Some potential future research directions include:
- Investigating the effects of other factors, such as pH and ionic strength, on the rate of crystal violet fading.
- Developing a more detailed kinetic model for the fading of crystal violet.
- Using the kinetics of crystal violet fading to develop new methods for measuring the concentration of hydroxide ions.
Question Bank: Kinetics Of Crystal Violet Fading Lab
What is the purpose of the Kinetics of Crystal Violet Fading Lab?
The Kinetics of Crystal Violet Fading Lab aims to investigate the factors that influence the rate of fading of crystal violet, a dye commonly used in various applications, and to understand the underlying mechanisms governing this process.
What are the key findings of the Kinetics of Crystal Violet Fading Lab?
The lab reveals that the fading rate of crystal violet is influenced by factors such as temperature, pH, and the presence of catalysts. The findings provide valuable insights into the dynamics of chemical reactions and the interplay of factors that affect their rates.
What are the applications of the findings from the Kinetics of Crystal Violet Fading Lab?
The findings from this lab contribute to the advancement of knowledge in reaction kinetics and have implications for various fields, including analytical chemistry, dye chemistry, and photochemistry. They can aid in optimizing industrial processes, developing new analytical methods, and understanding the behavior of dyes in different environments.


